Platinum Partitioning during core formation (Médard et al. 2015)
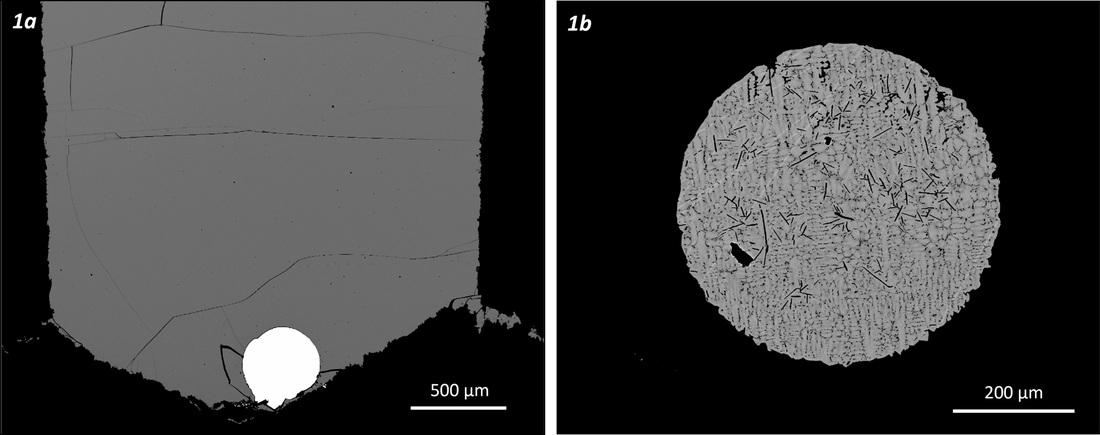
Highly Siderophile Elements (Au, Re, Ru, Rh, Pd, Os, Ir, Pt) have a strong geochemical affinity for metals and are thus excellent tracers for core formation processes in the terrestrial planets. For most HSE, however, partitioning data are scarce and most metal-silicate partition coefficients are derived from solubility experiments in iron-free systems which may or may not apply to the formation of an iron-rich core. Experiments at the low oxygen fugacitites relevant for core formation have also been limited by the presence of small metal particles, or "nanonuggets", with estimated sizes between 50 nm and 10 µm, that result in spikes or broad hills and highly unstable count rates during LA-ICP-MS analyses. The origin of these nanonuggets has been a matter of heated debate and they have been variably interpreted as (1) quench phenomena, or (2) equilibrium particles resulting from physical processes during the experiment. Whether these nanonuggets are considered to be present at run temperature (and thus excluded from the bulk concentration) or quench phases (and thus re-integrated into the bulk concentration) results in orders of magnitude of variation in partition coefficients for platinum group elements.
Fig. 1. Backscattered electron images of metal/silicate partitioning experiments
High-pressure, high-temperature experiments have been performed at 0.5-2.0 GPa and 1360-2100 °C to investigate the partitioning of Pt between a silicate melt and a metallic melt. Our experiments indicate that nanonuggets encountered in previous studies are experimental artifacts, formed at high temperature by oversaturation caused by high oxygen fugacity during the initial stages of an experiment. Experiments at high-acceleration using a centrifuging piston-cylinder show that nanonuggets can be removed by gravity during the experiment. Formation of nanonuggets can also be avoided by using initially reduced starting materials. The presence of iron is also a key element in reducing the formation of nanonuggets.
Fig. 2. The centrifuging piston-cylinder at ETH Zürich
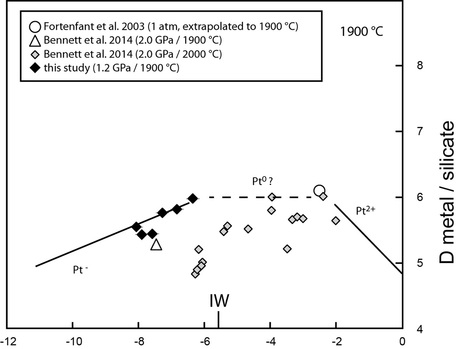
Our nanonugget-free data are broadly consistent with previous nanonuggets-filtered data, and suggest that under the reduced oxygen fugacity conditions expected for core formation processes (below IW-2), Pt is dissolved as an anionic species (possibly Pt- or Pt2-), instead of the more common Pt2+ species present at higher fO2. Calculations indicate that the Pt content (and by extension the Highly Siderophile Elements content) of the Earth’s mantle cannot be explained by equilibrium partitioning during core formation, requiring further addition of HSE to the mantle. The mass of the late accreted material is approximately 0.4% of the total mass of the Earth (or 0.6% of the mass of the mantle).
Further experiments are underway to determine the oxydation state and quantify the partitioning at low oxygen fugacity.
Further experiments are underway to determine the oxydation state and quantify the partitioning at low oxygen fugacity.
Fig. 3. Evolution of Pt partition coefficients with oxygen fugacity at 1900 °C
Fe partitioning and oxygen fugacity in high-pressure experiments (Médard et al. 2008)
We have perfected an experimentally calibrated model (Grove 1981) to extract the oxygen fugacity from the composition of silicate melts and equilibrium Pt-Fe alloys in high-temperature and high-pressure experiments. This technique allows to measure oxygen fugacity in experiments on magmatic processes by adding small pieces of Pt or Pt-Fe alloy to the experiment. A first model, developped for solid Pt-Fe alloys has been subsequently improved to be used for liquid Pt-Fe and Pt-Fe-C alloys (an excel spreadsheet for the Pt-Fe solid alloys can be downloaded here).
Using this method, we have experimentally determined the oxygen fugacity in experiments performed in Pt-graphite double-capsules, a container commonly used in high-temperature, high-pressure experimental petrology, particularly for anhydrous experiments relevant to primitive basaltic magmas and mantle melting. Oxygen fugacity in our piston-cylinder experiments using Pt-graphite capsules is CCO-0.7 (IW+1.5, QFM-2.2) at 1.5 GPa and 1360 °C. Comparison with other estimates and thermodynamic calculations indicate that a value of CCO-0.8 ± 0.3 can be used as a first approximation at least over the P-T range relevant for MORB and OIB magma generation (0.5–3.0 GPa, 1100–1500 °C). Under those conditions, the amount of Fe3+ in silicate phases (pyroxenes, olivine, glass) and spinel is negligible (Fe3+/ΣFe < 0.05) and would not significantly affect thermodynamic properties. Oxygen fugacities higher than CCO cannot be achieved using Pt-graphite or graphite only capsules, but fO2 can be tuned to lower values by using small pieces of Pt-Fe alloys. The potential range of fO2 that can be reached in graphite or Pt-graphite capsules with this technique is CCO to CCO-4. Lower values, down to IW-10 (CCO-11), can be obtained by addition of Si metal to the experimental charges.
Using this method, we have experimentally determined the oxygen fugacity in experiments performed in Pt-graphite double-capsules, a container commonly used in high-temperature, high-pressure experimental petrology, particularly for anhydrous experiments relevant to primitive basaltic magmas and mantle melting. Oxygen fugacity in our piston-cylinder experiments using Pt-graphite capsules is CCO-0.7 (IW+1.5, QFM-2.2) at 1.5 GPa and 1360 °C. Comparison with other estimates and thermodynamic calculations indicate that a value of CCO-0.8 ± 0.3 can be used as a first approximation at least over the P-T range relevant for MORB and OIB magma generation (0.5–3.0 GPa, 1100–1500 °C). Under those conditions, the amount of Fe3+ in silicate phases (pyroxenes, olivine, glass) and spinel is negligible (Fe3+/ΣFe < 0.05) and would not significantly affect thermodynamic properties. Oxygen fugacities higher than CCO cannot be achieved using Pt-graphite or graphite only capsules, but fO2 can be tuned to lower values by using small pieces of Pt-Fe alloys. The potential range of fO2 that can be reached in graphite or Pt-graphite capsules with this technique is CCO to CCO-4. Lower values, down to IW-10 (CCO-11), can be obtained by addition of Si metal to the experimental charges.
Relevant publications
Médard E, Schmidt MW, Wälle M, Keller NS, Günther D (2015) Platinum partitioning between metal and silicate melts: core formation, late veneer and the nanonugget issue. Geochimica et Cosmochimica Acta 162: 183-201, doi: 10.1016/j.gca.2015.04.019.
Médard E, McCammon CA, Barr JA, Grove TL (2008) Oxygen fugacity, temperature reproducibility, and H2O content for nominally dry piston-cylinder experiments using graphite capsules. American Mineralogist 93: 1838-1844, doi: 10.2138/am.2008.2842.
my latest results have been presented at the 48th LPSC conference, and will be submitted soon for publication:
Médard E, Martin AM, Righter K, Malouta A, Lee C-T (2017) Anionic Pt in silicate melts a t low oxygen fugacity: speciation, partitioning and implications for core formation processes on asteroids. 48th Lunar and Planetary Science Conference, Abstract #2582, The Woodlands TX, March 20-24.
Médard E, McCammon CA, Barr JA, Grove TL (2008) Oxygen fugacity, temperature reproducibility, and H2O content for nominally dry piston-cylinder experiments using graphite capsules. American Mineralogist 93: 1838-1844, doi: 10.2138/am.2008.2842.
my latest results have been presented at the 48th LPSC conference, and will be submitted soon for publication:
Médard E, Martin AM, Righter K, Malouta A, Lee C-T (2017) Anionic Pt in silicate melts a t low oxygen fugacity: speciation, partitioning and implications for core formation processes on asteroids. 48th Lunar and Planetary Science Conference, Abstract #2582, The Woodlands TX, March 20-24.
Proudly powered by Weebly